Test Results Frequently Asked Questions
Why am I getting two different readings with the same water?
This should not happen so long as you followed the directions for both tests and the test strips have not deteriorated. The LabTech® test strips have been lab-tested to assure accurate test results when properly used.
The test results produced a color that is not on the color chart. What does that mean?
If a color reaction does not match the standards on the color chart, estimate the result as a mid-point between two color standards. Example: The pad color is darker than the 100ppm but lighter than the 250ppm standard for hardness. The result would be estimated at the mid-point, or 175ppm.
How does the Coliform Bacteria test work?
Our Sample Procedure takes an important extra step in removing the chlorine from your water sample before testing. Chlorine, which exists in most municipal water systems, can suppress the bacterial reaction in the test. Removing the chlorine before the test eliminates chlorine interference.
The Coliform Test tablet in the glass tube (4890) contains nutrients to support the growth of
coliform bacteria, a gelling substance, and a pH indicator.
If coliform organisms are detected in the sample:
-
Gas will be generated as a result of the bacteria metabolizing the nutrients in the tablet.
-
The gas will be trapped in the gelling substance and cause the gel to rise in the tube. Bubbles will be present in the gel.
-
The pH indicator will change from red to yellow.
Both the gel rising to the surface and the color change must occur to indicate the existence of coliform bacteria.
Note: Some water samples may cause a shift in the pH indicator to the yellow color when coliforms are not detected. However, in this case no gas bubbles will be generated, and the solution will be clear with the gel will remain at the bottom of the tube. In this case, coliform bacteria have not been detected.
Why do my Lead or Pesticide test strips show different results than the instructions?
Some tests may have incomplete lines which do not go all the way across the strip. This is sometimes caused by small particles in the water. When these particles lodge in the bottom of the strip, they can prevent the flow of water above the particle and the formation of the result lines. The rest of the test typically flows as intended, and the result can be interpreted by comparing the visible parts of the blue lines.
Here are some examples with interpretation of the result:
Negative Lead/
Inconclusive Pesticide |
 |
|
Both Tests Negative |
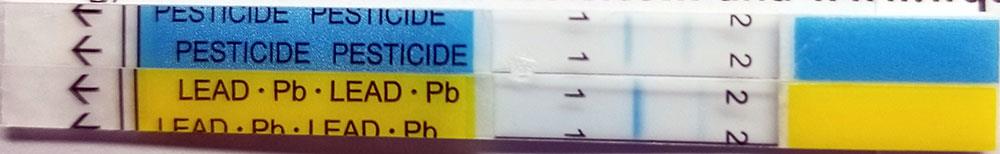 |
Positive Lead/
Negative Pesticide |
 |
|
Both Tests Inconclusive |
|
What is Hydrogen Sulfide in the water?
Hydrogen sulfide is an insoluble gas often found in water resulting from being trapped in water underground. When the water is pumped to the surface, the smelly hydrogen sulfide gas escapes and is therefore easy to detect. Concentrations greater than 1 ppm smell like “rotten eggs” and are corrosive to plumbing. The odor may be noticeable only when the water is initially turned on or when hot water is running. Heat forces the hydrogen sulfide gas into the air, which may cause the odor to be particularly offensive in the shower.
Hydrogen Sulfide can also form in your hot water heater where the magnesium corrosion control rod present in many electric hot water heaters chemically converts naturally occurring sulfates in water to hydrogen sulfide. If this is the sole source of hydrogen sulfide, only hot water will have this odor.
If the hydrogen sulfide gas is being produced by an active colony of sulfur-reducing bacteria, this must be determined and eliminated by shock chlorination. However, if you detect the smell of hydrogen sulfide only from the hot water faucet, your water heater may be causing the problem. In this case, shock treatment is not warranted, and the problem can be eliminated or minimized by replacing the magnesium rod with one made of aluminum or zinc, preferably by a licensed plumber. Specialized lab testing using gas stabilization techniques is necessary to determine the exact cause.
What is Iron Bacteria in the water?
Often confused with Hydrogen Sulfide because of similar water smells, Iron Bacteria poses no health risk, but makes water unsightly and causes an unpleasant sewage or rotten vegetation odor and taste. It is the result of elevated levels of iron or manganese in water wells often giving rise to the growth of iron bacteria. Iron may be present naturally in the rocks surrounding the well water supply or introduced in the well during well drilling or pump installation operations.
Iron bacteria produces a filamentous, slimy deposit that can clog filters and plumbing components. Laboratory testing for iron bacteria is not necessary. Its presence can be confirmed using an easy observation detection method.
Iron bacteria is difficult to fully eradicate. Shock chlorination is the most practical method to kill or control iron bacteria.